
Lone pair - lone pair > lone pair - bond pair > bond pair - bond pair In general, the strengths of electron pair repulsions decline in the order This is the result of differences in the strengths of repulsions between electrons involved in bond formation and those that are not. Bond angles in these molecules will often deviate from the ideal angles of the electron domain geometries. However, the resulting shape for molecules with lone pairs on the central atom will be based on the electron domain geometries we have just seen. Keep in mind that the shape of a molecule refers to the geometrical arrangement of its atoms, not the domain geometry of its electron pairs. In these cases, the electron domain arrangements will be the same for the total number of electron pairs, but the molecular shape will be different. In many molecules some pairs of valence electrons around the central atom may be non-bonding lone pairs. Consider BeCl 2, for which the Lewis dot structure is To illustrate the approach, let us consider a number of molecules in which all of the valence electrons about the central atom are engaged in bond formation with outer-lying (pendant) atoms. However, assuming that such orbitals are available, we can determine for various numbers of pairs of electrons about a central atom the most favorable electron pair geometry to minimize electron-electron repulsions. At this point we will not be concerned with the identity of the spatially oriented orbitals.
The approach is remarkably accurate in predicting the basic shapes of molecules of non-transition elements, although some details of bond angle and bond length are not well explained by electron repulsion alone. The fundamental premise of this theory can be stated as follows:Įlectrons in bonded atoms occupy spatially oriented orbitals in such a way as to minimize electron-electron repulsions arising mainly from electrostatic (coulombic) forces. This approach, which was originated by Nyholm and Gillespie in the 1950's, has become known as the Valence Shell Electron Pair Repulsion Theory, or VSEPR. One of the most successful approaches to predicting the shapes of molecules is based almost solely on considerations of how best to minimize electron-pair repulsions about a central atom. Knowing the shape of a molecule enables us to predict whether or not it has an electrical polarity, which is an important property determining how the species interacts with other molecules. This method is called the Valence Shell Electron Repulsion Theory, or VSEPR for short. Although the Lewis structures themselves do not convey shape information, they can be used as the starting point for applying a conceptually simple but powerful approach to predicting molecular geometries.
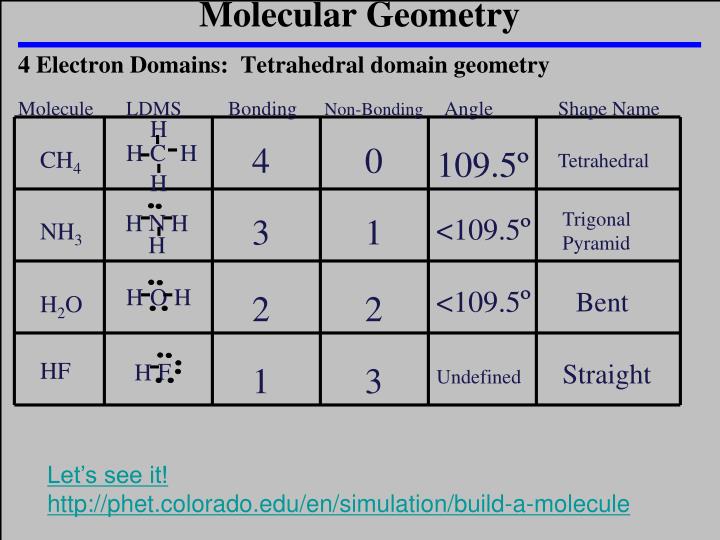
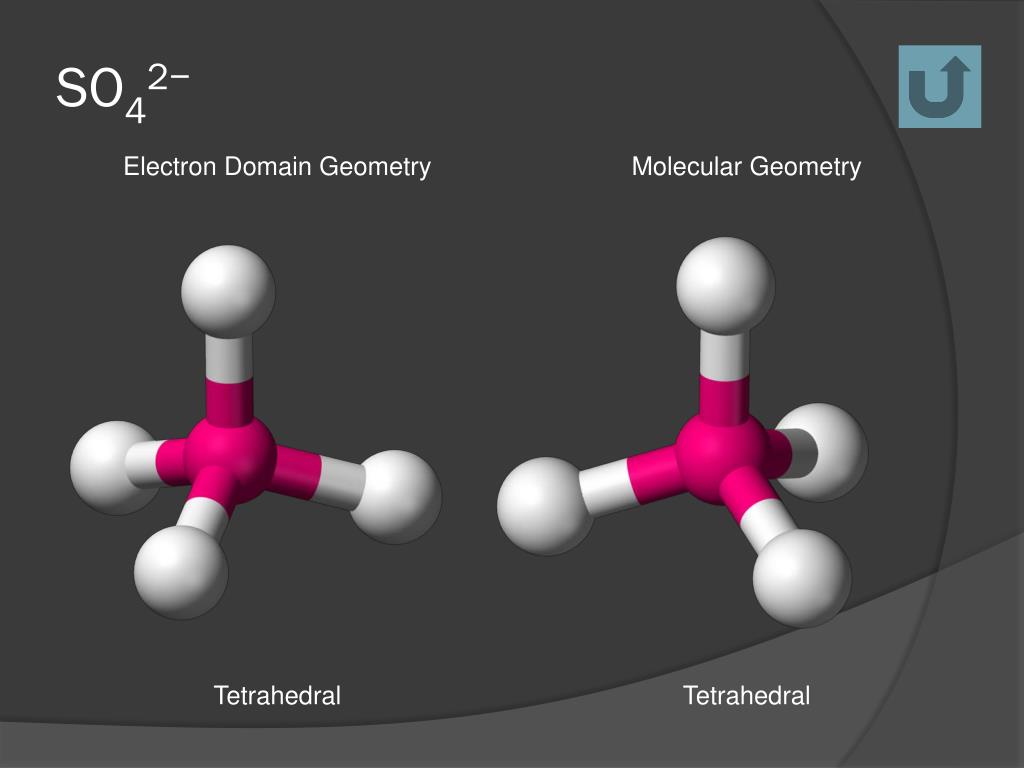
These molecular shapes are very important to understanding how molecules interact with each other, both chemically and physically.

Know how bond polarity and molecular shape combine to make a molecule polar or nonpolar.Ĭontrary to the impression that Lewis structures may give, many molecules have three-dimensional geometries.Understand how bond pairs and lone pairs about a central atom interact to produce the molecular shape.Know the expected geometries for one through six electron domains about a central atom.Understand the basis of the VSEPR theory.


 0 kommentar(er)
0 kommentar(er)
